NICE publishes second draft guidance on Efgartigimod for treating generalised myasthenia gravis

In September we shared the news that NICE had published draft guidance on the use of the treatment efgartigimod for generalised myasthenia gravis, which didn’t recommend it for NHS use.
NICE consulted on this draft guidance and a second committee meeting took place on 16 November. Usually, publication of final guidance on the treatment being appraised would follow this, but in this case, NICE has taken the rare step of publishing a second draft guidance with a third committee meeting scheduled for May 2024.
This second draft guidance still finds that efgartigimod should not be recommended for NHS use, but the fact that there will be a third committee meeting suggests NICE feels some of the issues around the treatment that led to this position could be addressed through further discussion and evidence gathering.
What does the draft guidance say?
This second draft guidance repeats the conclusions of the first that:
-
Efgartigimod is not recommended, within its marketing authorisation, as an add-on to standard treatment for generalised myasthenia gravis in adults who test positive for anti-acetylcholine receptor antibodies.
-
This recommendation is not intended to affect treatment with efgartigimod that was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before the guidance publication, until they and their NHS clinician consider it right to stop.
What does this mean?
If accepted, this means people not currently receiving efgartigimod through the NHS in England will be unable to access it. However, people already being treated with efgartigimod will be able to continue to do so until their doctor considers it's no longer necessary.
The NICE appraisal consultation document can be read in detail here and is now open for consultation.
The draft guidance acknowledges that ‘clinical trial evidence suggests that efgartigimod plus standard treatment improves symptoms and people’s ability to carry out their normal activities compared with standard treatment alone’ but highlights uncertainties that led the appraising committee to conclude it wasn’t clear efgartigimod was cost-effective enough to be recommended for NHS use.
Some of the remaining areas of uncertainty include to what degree evidence gathered from clinical trials applies to the group of patients who the company wants to make the treatment available to; the degree to which efgartigimod would reduce the need for ‘maintenance intravenous immunoglobulin (IVIg)’, a treatment that can be used to boost antibody levels in people with myasthenia gravis; how the possible placebo effect from clinical trials has been factored into assessments of the treatment’s effectiveness; and the impact of myasthenia gravis on carers.
Our response and next steps
We’re disappointed and frustrated that some of the uncertainties highlighted in the first draft guidance remain unresolved. We’re particularly concerned that the impact of myasthenia gravis on carers is still considered uncertain, when much of the evidence we, patient experts and the charity MyAware, have put forward has focused on this issue.
We’ve received compelling testimony from people who have received efgartigimod through an Early Access to Medicines Scheme (EAMS) about its positive impact on them and their families, and we’re calling on all parties to work together to address the uncertainties that remain −we’ve met with NICE and the company since the last committee meeting.
While delays in securing wider access to efgartigimod are concerning, the fact that there will be a third NICE committee meeting is a positive sign that they feel the uncertainties could still be addressed and a positive recommendation might be made. We’ll continue to engage in the formal NICE process and will work with all parties in the hope that the process can be sped up.
How you can share your views
If you’re currently receiving efgartigimod and are affected by this news, we’re keen to hear from you so we can include your views in our work. Email our Campaigns teams.
We will again be making a joint consultation submission with MyAware. You can make your own direct submission to the process through the NICE website.
If you’ve been affected by this announcement, contact us on 0800 652 6352, Monday to Friday, 10am-2pm, or email: info@musculardystrophyuk.org
What are the key takeaways from the Government’s Autumn Statement?
Last week, Wednesday 22 November, the Chancellor of the Exchequer Rt Hon Jeremy Hunt MP, delivered the Autumn Statement which provides an update on the government’s economic plans. With an upcoming general election anticipated in the new year we were eagerly anticipating the government’s announcements to understand the direction they would set for 2024.
Disability History Month: your school experiences of Physical Education
Double your Donation 2023


Why is this important?
More than 110,000 adults and children in the UK live with a muscle weakening or wasting condition.
This can be exhausting, stressful and lonely, with endless medical appointments, physiotherapy, treatments, and respiratory support.
Progressive conditions get worse over time. They can cause difficulty walking, swallowing, breathing complications, pain, heart problems and failure. Life can be more challenging or cut short.
That’s why for over 60 years we’ve been funding groundbreaking research and life-changing support.
How your donation will make double the difference
We have made advances that would have been unthinkable just 10 years ago and with your help we can go even further, faster. We fund groundbreaking research with the aim of finding treatments for all types of muscle wasting and weakening conditions.
In recent years there have been great advances, with 13 new treatments either available on the NHS or being assessed. While this progress is exciting, there are still over 50 conditions where people don't have access to a treatment, and still far too many people without access to a diagnosis.
By funding pioneering research, we change the future of people living with muscle wasting conditions.
Henry aged six lives with Becker muscular dystrophy

We know that MDUK will pull us and Henry through. And with them funding research into so many conditions, it feels like there's hope for the future too; not just for Henry, but everyone with a muscle wasting condition. Please, will you donate now and spread that hope and support to even more families?
Michelle, Henry's mum.
Our Double Your Donation campaign means you can make twice the impact through just the one donation. Donate on the page below from 28 November and together we can change the future of muscle wasting conditions.

Reflection on the Annual Report
Impact Report 2022-2023
Skin cells' potential to form muscle cell models
Celebrating a landmark day with the 2,000th Changing Place toilet at Leeds United
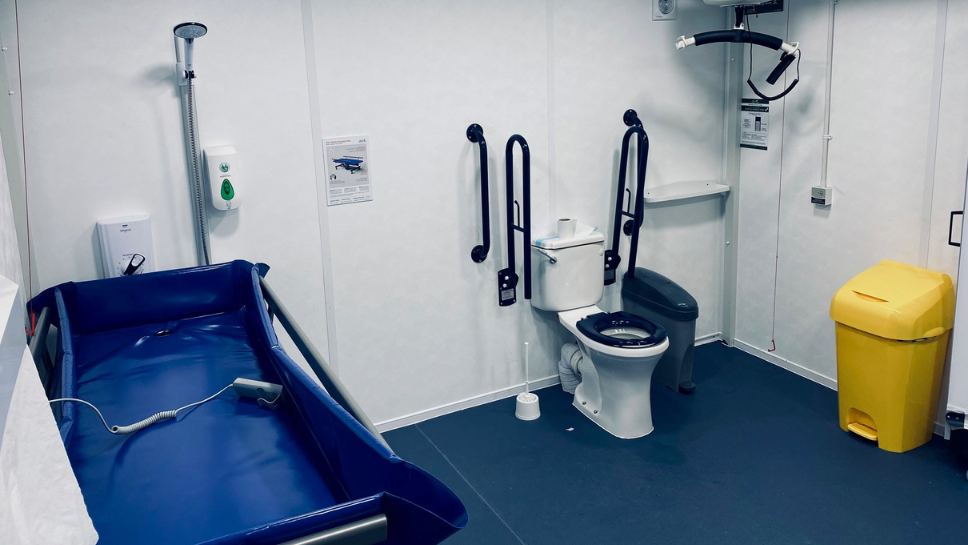
Changing Places toilets have more space than standard accessible toilets, and include essential equipment such as a ceiling hoist, peninsular toilet, and adult-sized changing bench, making them accessible to a wider range of people.
As co-chair of the Changing Places Consortium with PAMIS, we promote the installation of new CPTs in public spaces and a wide range of venues and support those campaigning for more CPTs. We also register CPTs that meet required standards and maintain the Changing Places website and map. Other consortium members include the Centre of Accessible Environments (CAE) and Martin Jackaman.
We’re incredibly proud to see how far the campaign has come. Registrations of Changing Places toilets began back in 2008, with just 13 CPTs registered in that first year. The Changing Places message has spread far and wide, bringing increasingly more venues into the inclusive fold.
Last year 162 new CPTs were registered across the UK, and this year looks to be on-track to beat that again.
There’s no denying that the Leeds area demonstrates a fantastic commitment to accessibility and inclusion with a high number of registered CPTs. Bairbre McKendrick, Access Officer for Leeds City Council said:
I would like to congratulate Vince and Helen (all the team) at Leeds United for the new facilities they have recently delivered which will make match days at Elland Road more inclusive. The new Changing Places toilet will mean more disabled people can now join their friends and families and enjoy live football as part of the Leeds United Community.
To have reached the 2,000th registered CPT is an amazing achievement, and a testament to the determination and dedication of the campaigners, as well as the commitment to inclusion from the venues who appear on the Changing Places toilets map.
A huge congratulations to Leeds United Football Club – your Changing Places toilet will truly change lives.
While it’s wonderful to have reached this milestone, the work on the Changing Places toilet campaign is far from over. Until Changing Places toilets are installed in all public spaces, the consortium will continue to work closely with campaigners, venues, suppliers, and funders as we work towards a fully inclusive society.
Myasthenia gravis treatment Efgartigimod not recommended for use in Scotland

The Scottish Medicines Consortium (SMC) has completed its assessment of efgartigimod alfa (Vyvgart®) as an add-on to standard therapy for the treatment of adults with generalised Myasthenia Gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive. The assessment has found that the treatment should not be used in Scotland. The SMC advises NHS Boards and Area Drug and Therapeutic Committees (ADTCs) on the use of treatments in NHS Scotland.
The SMC advice acknowledges that in a phase III study, efgartigimod significantly improved Myasthenia Gravis Activities of Daily Living (MG-ADL) responder rate compared with placebo in patients with gMG who were AChR antibody positive; but found that argenx, the company that produce the treatment, did not present sufficiently robust clinical and economic analysis to gain acceptance by SMC.
A number of patients in Scotland have already begun treatment with efgartigimod through argenx’s free-of-charge (FOC) scheme. The company has confirmed that all patients who have received the treatment will continue to do so for as long as they need it. However, no new patients will be able to enrol on this scheme.
argenx has also confirmed that it is continuing to explore options to make efgartigimod available to patients in Scotland and that it has plans to resubmit to the SMC in the near future. Under SMC processes, companies may request a meeting if their treatment has not been recommended. The aim of the meeting is to help the company understand why their medicine has not been accepted and determine the nature and focus of any resubmission or independent review panel (IRP).
In England, the second NICE (The National Institute for Health and Care Excellence) Appraisal Committee Meeting for efgartigimod is taking place on Thursday 16 November 2023.
If you have any questions or would like more information about our work on access to treatments, email campaigns@musculardystrophyuk.org. Our helpline is available Monday-Thursday 10am-2pm on 0800 652 6352.
If you are a patient in Scotland already receiving efgartigimod and have any questions about the FOC scheme, speak to your treating physician who will use the appropriate channels to discuss this with argenx.
