We fund pioneering research for better treatments to improve people’s lives today, and to transform those of future generations.
Stem cells
Our bodies are made up of many different types of cells that are specialised to perform particular functions. For example, the individual fibres that make up our muscles are specialised for muscle contraction.
Stem cells are distinct from other cells in our body in that they are unspecialised. They have the ability to develop into many different types of cells through a process called ‘differentiation’. They are also able to self-renew, which means they can keep dividing and producing identical copies of themselves. These properties therefore make them attractive as a potential therapeutic option for muscle wasting conditions.
Stem cells are important in early life and growth, as they develop into the cells that make up all of our tissues and organs. But their role doesn’t end there. They are continually working throughout our lives to ensure we have all the cells we need. They are essential to the maintenance of tissues such as skin, gut and blood that undergo continuous turnover. They are also vital in maintaining muscle, which can be built up according to the body’s needs and can often get damaged during physical exertion.
There are two main types of stem cells found naturally inside the body:
- embryonic stem cells – as their name suggests, these stem cells originate from an embryo. They supply new cells to the embryo as it grows and develops into a baby. Embryonic stem cells are pluripotent, which means they can develop into any type of cell.
- adult (somatic) stem cells – these not only supply new cells as a person grows, but also replace cells that get damaged. Somatic stem cells are multipotent, which means they can only change into certain cell types. For example, muscle stem cells (satellite cells) specialise into muscle cells. However, research has found that some adult stem cells are more versatile than previously thought. Stem cells from blood vessels (mesoangioblasts) and even from fat tissue (adipose stem cells) are capable of becoming muscle cells under certain growth conditions (see figure 1).

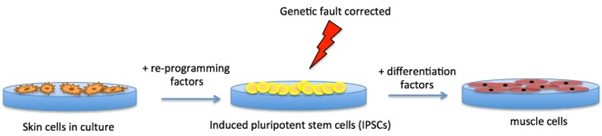
It is also possible to manufacture stem cells in the laboratory by adding a cocktail of ‘reprogramming’ factors to specialised cells such as skin cells. These specialised cells then convert to induced pluripotent stem cells (iPSCs), which can subsequently be converted into any type of cell. iPSCs are similar to embryonic stem cells in this way.
From a therapeutic perspective, iPSCs can be produced from a patient’s own cells, which makes them useful tools for studying the treatment of human diseases. And, because they’re made from a person’s own cells, they can be used to create cells that can be transplanted back into the person without the risk of immune rejection. For example, iPSCs from someone with a genetic muscle wasting condition could be genetically ‘corrected’ outside of the body using a gene therapy or genome-editing approach, allowed to differentiate into healthy muscle cells, which are then transplanted back into the body (see figure 2 below).
Stem cells found in skeletal muscle are called satellite cells and their function is disrupted in some muscle wasting conditions. This is because the muscle gets damaged easily and there is a continuous cycle of muscle degeneration and regeneration. The satellite cells cannot keep up with the demand for new muscle fibres and eventually become exhausted and ineffective, which ultimately leads to muscle wasting.
The underlying genetic change can also directly affect satellite cell function. For example, the mutation that causes facioscapulohumeral muscular dystrophy (FSHD) leads to the activation of a protein called DUX4, which is toxic to satellite cells. The loss of dystrophin in Duchenne muscular dystrophy may also affect the division of satellite cells.
Stem cell therapy is the transplantation of stem cells into patients, using either their own cells or those of a donor. This therapy has the potential to benefit people with muscle wasting conditions, as it could encourage the growth of new muscle fibres in damaged muscle. However, to date, no stem cell therapy has been proven effective in treating these conditions. There have been few clinical trials, which have unfortunately been unsuccessful. One of the main reasons is the difficulty in delivering these cells to the muscle.
Researchers need to overcome several challenges before stem cell therapy could become an approved treatment for people with muscle wasting conditions. These include:
- growing large volumes of stem cells in the laboratory, without losing their regenerative properties
- finding a suitable delivery mechanism, so that the stem cells reach all of the affected muscle
- improving the integration of stem cells into muscle (engraftment), so that they can successfully make new muscle cells
- preventing the body’s immune system from rejecting the transplanted stem cells.
Transplanting a patient’s own stem cells is one way to reduce the risk of immune rejection. However, for genetic conditions such as muscular dystrophy, the underlying genetic mutation would still affect these cells and they may not function as well as stem cells from a healthy person. This means that a combination of gene and cell therapy may be required, by genetically correcting the stem cells in the laboratory before transplantation.
Alternatively, researchers could use donor or embryonic stem cells. However, as with an organ transplant, there’s a risk of immune rejection of the introduced cells. In this situation, there would need to be careful matching of the donor, and the recipient may need to take immunosuppressive drugs.
Online, you may come across clinics around the world offering stem cell therapies; it’s important to remember that almost none of these are approved. Current stem cell therapies that are approved by the US Food and Drug Administration (FDA) only target certain blood disorders and cancers. The European Medicines Agency (EMA) has also approved one that helps to repair part of the eye after injury. It is extremely important to test investigational products such as stem cell therapies in clinical trials and for regulators to assess them. This ensures the safety of patients, by only using drugs or treatments that are effective.
Although there is a lot of ongoing stem cell research for muscle wasting conditions, there is no consensus over which type of stem cell has the most potential as a therapy. Researchers are investigating a number of different types of stem cell, and each has its own pros and cons.
Satellite cells – which are found naturally inside the muscle – might seem an obvious choice for a stem cell therapy but there are a number of issues with them. When grown outside the body, satellite cells lose their regenerative capacity and so do not form large amounts of muscle when transplanted. They also rely on the host muscle environment to function properly, so they may only have limited effectiveness in a dystrophic muscle.

Our comprehensive resources cover a diverse range research topics. Whether you want to understand more about exon skipping, the intricacies of genetic therapies or animals in research, our hub offers valuable insights into the world of modern science.